Acid/Base Equilibria Notes Part 1: The 3 Acid/Base Definitions, Hydronium, Conjugate Acid/Base Pairs & their Relative Strengths March 23, ppt download
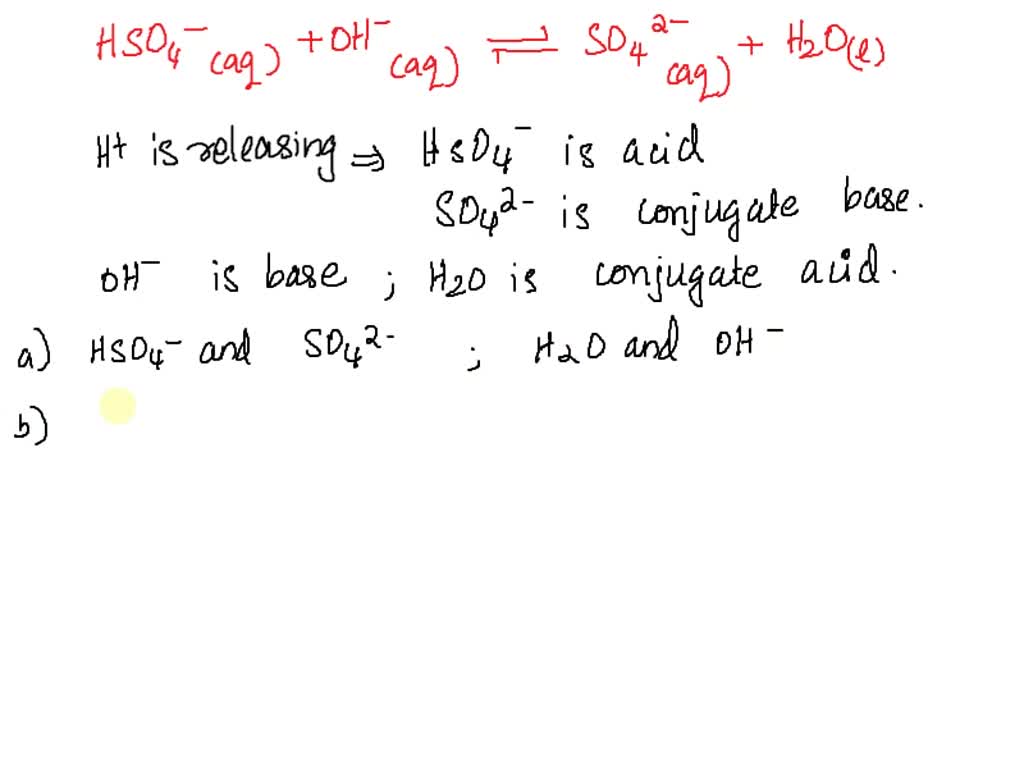
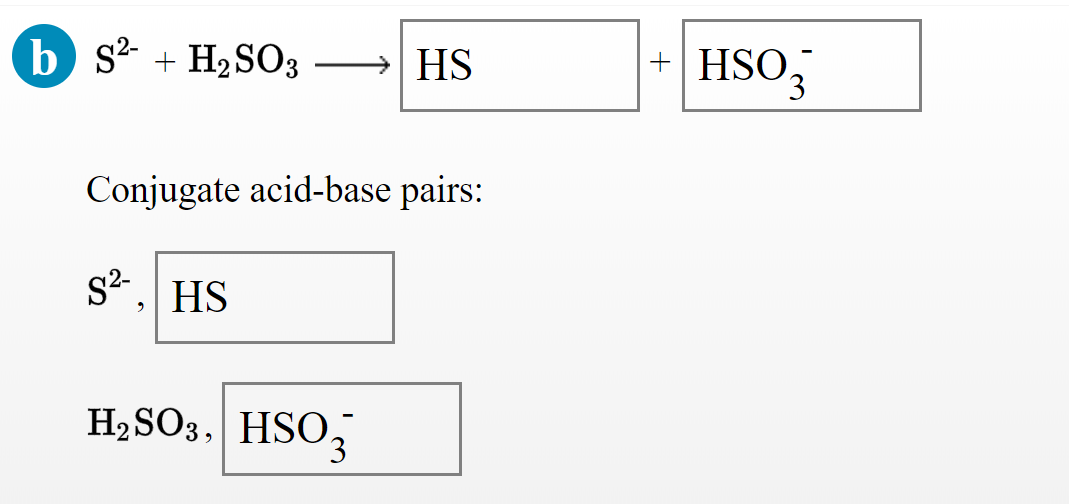
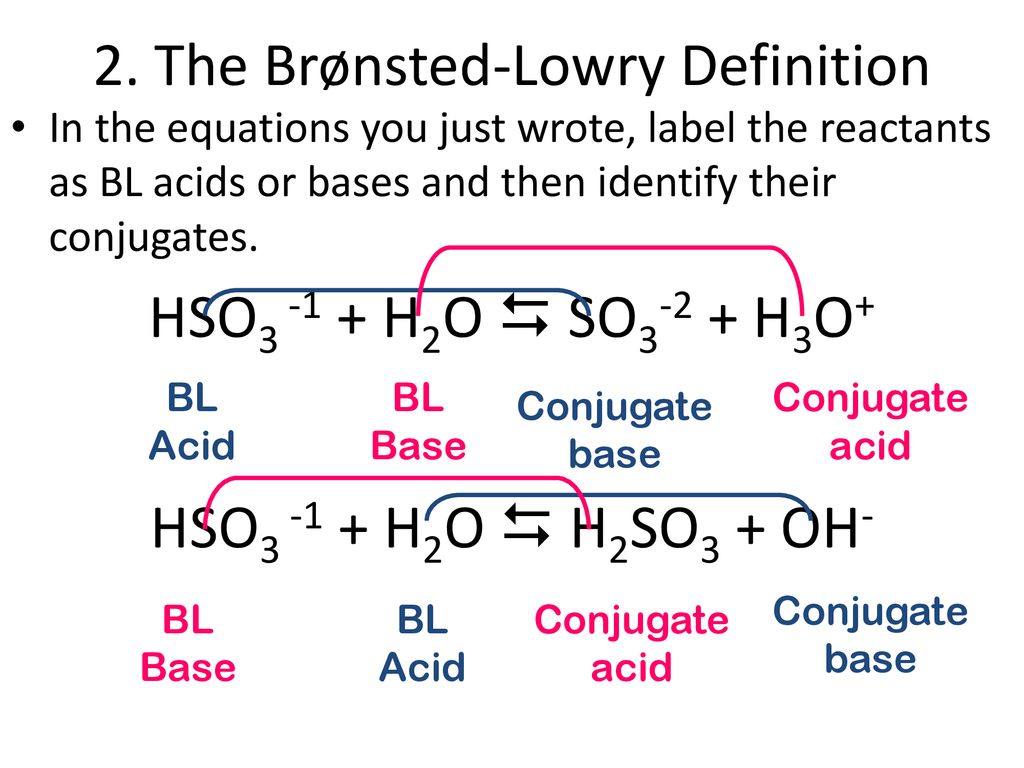
SOLVED: 'In the following reaction S032-(aq) H2o(e) == HSOz (aq) - OH (aq) SO32 - is a base and HSO3 is its conjugate acid SO32 base and H2O is its conjugate acid

Ammonia Catalyzed Formation of Sulfuric Acid in Troposphere: The Curious Case of a Base Promoting Acid Rain | The Journal of Physical Chemistry A

Acid/Base-Free Acyclic Anionic Oxoborane and Iminoborane Bearing Diboryl Groups | Inorganic Chemistry
![SOLVED: 22. Based on the Lewis structures of the reactants and product of the following equation, identify the Lewis acid and the Lewis base in it: I + [ 15 6 @ [+] SOLVED: 22. Based on the Lewis structures of the reactants and product of the following equation, identify the Lewis acid and the Lewis base in it: I + [ 15 6 @ [+]](https://cdn.numerade.com/ask_images/4fe6fd50e149442ba6d53d16107fe043.jpg)
SOLVED: 22. Based on the Lewis structures of the reactants and product of the following equation, identify the Lewis acid and the Lewis base in it: I + [ 15 6 @ [+]

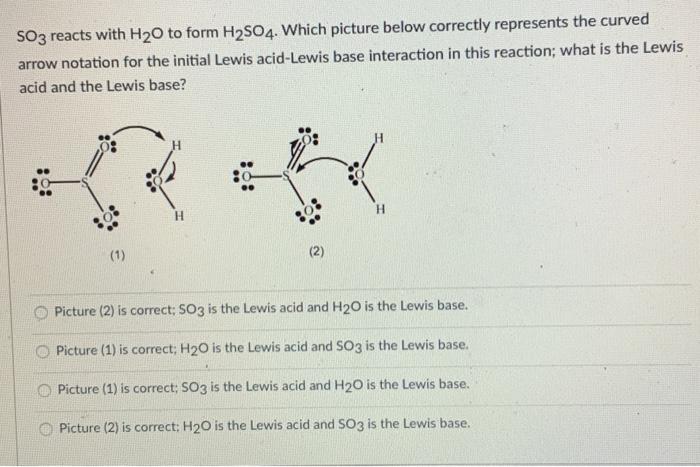
SOLVED: Q; in the reaction between H2O and SO3, SO3 acts as a Lewis acid while H2O act as a Lewis base true or false ?
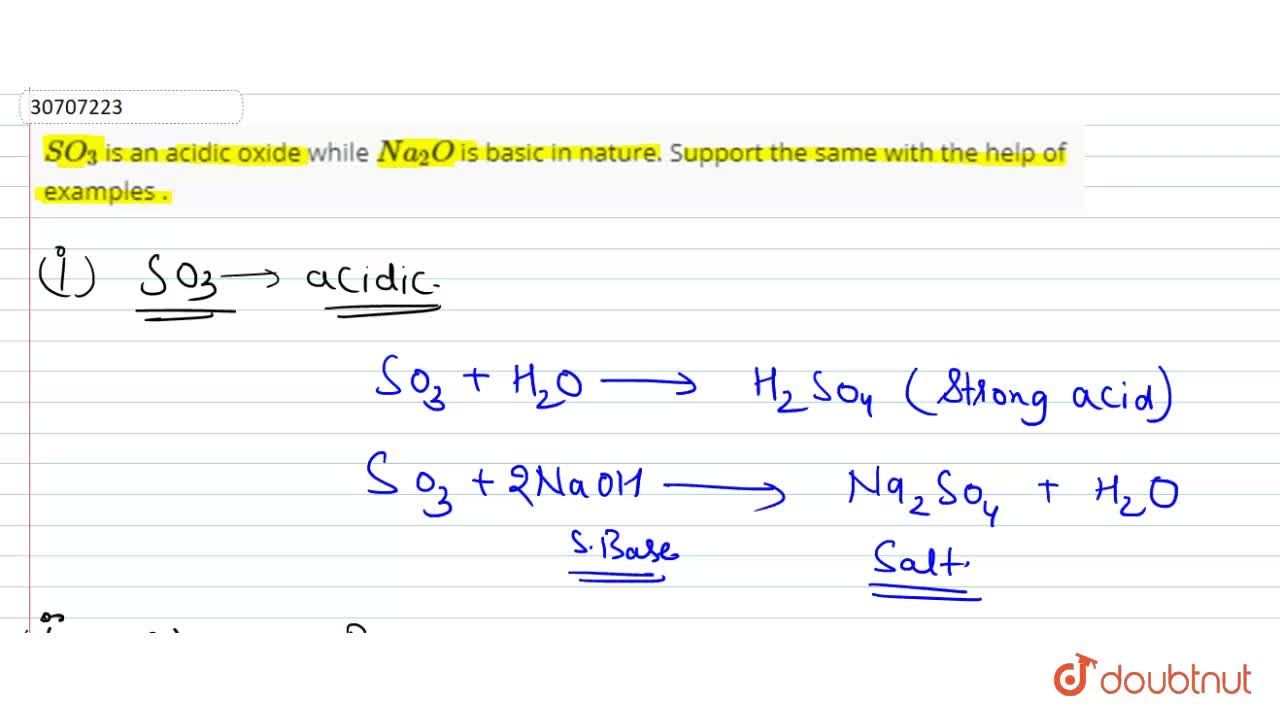
SO(3) is an acidic oxide while Na(2)O is basic in nature. Support the same with the halp of exaples .
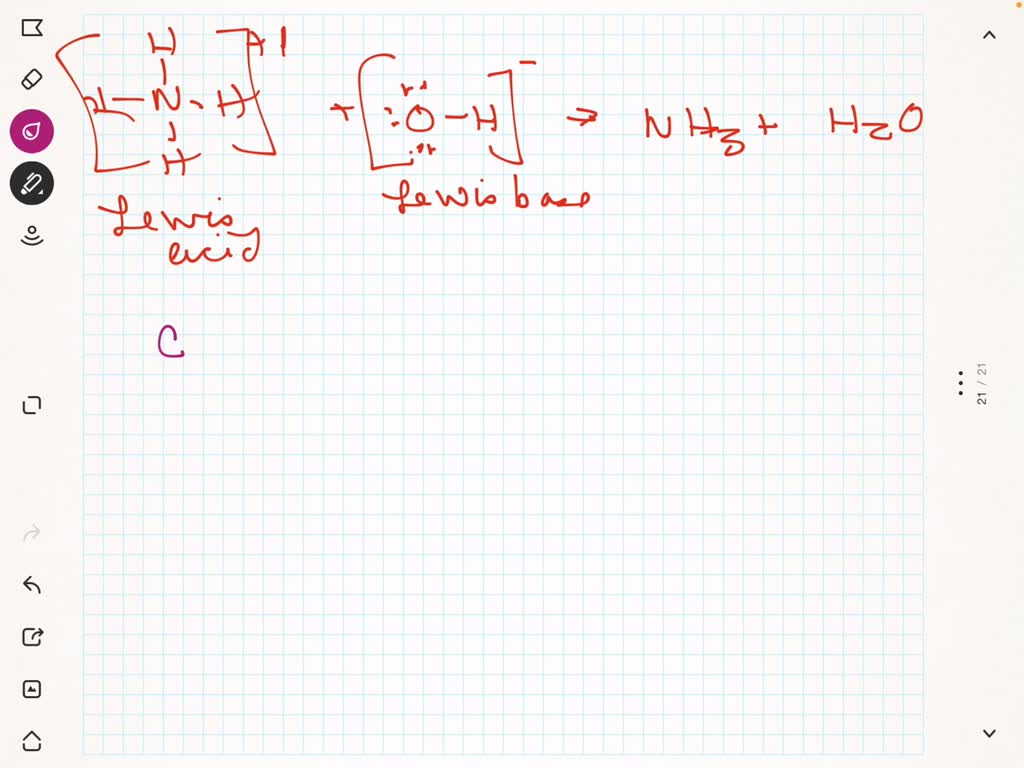
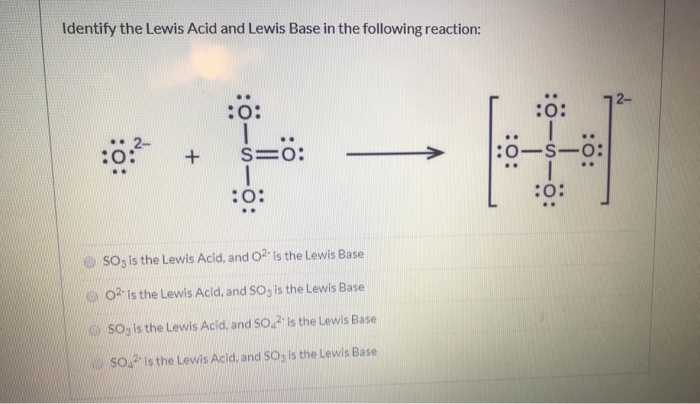
SOLVED: Answer the question below: 1: i) Identify the Lewis acids and bases in each of the following reactions: a. NH3 + BF3 → F3B NH3 b. H2O + SO3 → H2SO4

Formic Acid Catalyzed Hydrolysis of SO3 in the Gas Phase: A Barrierless Mechanism for Sulfuric Acid Production of Potential Atmospheric Importance | Journal of the American Chemical Society