Proposed mechanisms for the DBU catalyzed urea-synthesis reaction from... | Download Scientific Diagram
![The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters](https://pubs.acs.org/cms/10.1021/acs.orglett.5b02398/asset/images/medium/ol-2015-02398j_0006.gif)
The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters
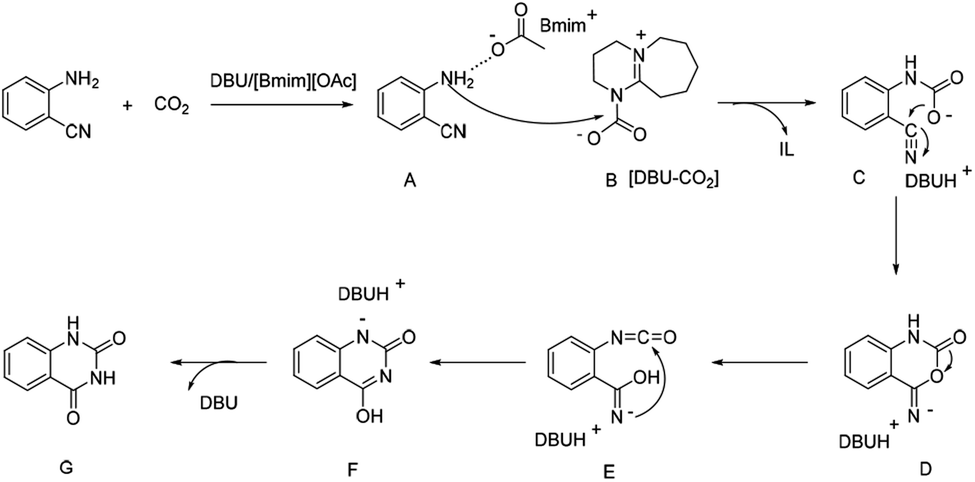
DBU coupled ionic liquid-catalyzed efficient synthesis of quinazolinones from CO 2 and 2-aminobenzonitriles under mild conditions - RSC Advances (RSC Publishing) DOI:10.1039/D0RA00194E
![1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU): A Versatile Reagent in Organic Synthesis | Bentham Science 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU): A Versatile Reagent in Organic Synthesis | Bentham Science](https://www.eurekaselect.com/images/graphical-abstract/coc/19/9/0005D.gif)
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU): A Versatile Reagent in Organic Synthesis | Bentham Science

SciELO - Brasil - Mechanistic Investigation of DBU-Based Ionic Liquids for Aza-Michael Reaction: Mass Spectrometry and DFT Studies of Catalyst Role Mechanistic Investigation of DBU-Based Ionic Liquids for Aza-Michael Reaction: Mass Spectrometry
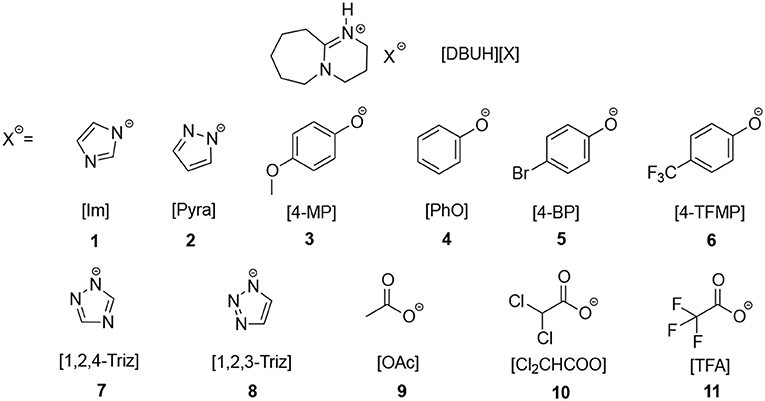
Frontiers | CO2 Absorption by DBU-Based Protic Ionic Liquids: Basicity of Anion Dictates the Absorption Capacity and Mechanism
![Controlled Reactivity of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) in the Selective Synthesis of 1‐(Bromoethynyl)arenes - Krishna Moodapelly - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library Controlled Reactivity of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) in the Selective Synthesis of 1‐(Bromoethynyl)arenes - Krishna Moodapelly - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/da071cf8-eee0-4e2f-8e22-5b6f6a0f91e7/adsc201601279-fig-5004-m.jpg)
Controlled Reactivity of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) in the Selective Synthesis of 1‐(Bromoethynyl)arenes - Krishna Moodapelly - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library
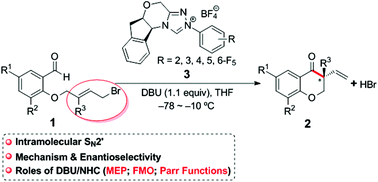
Theoretical study on the mechanism and enantioselectivity of NHC-catalyzed intramolecular SN2′ nucleophilic substitution: what are the roles of NHC and DBU? - Organic Chemistry Frontiers (RSC Publishing)

Structure of the PILs' precursors: DBU base (a) and the three acids... | Download Scientific Diagram
Investigating the Underappreciated Hydrolytic Instability of 1,8-Diazabicyclo[5.4.0]undec-7-ene and Related Unsaturated Nitrogen
![Figure 1 from 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) and microwave-accelerated green chemistry in methylation of phenols, indoles, and benzimidazoles with dimethyl carbonate. | Semantic Scholar Figure 1 from 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) and microwave-accelerated green chemistry in methylation of phenols, indoles, and benzimidazoles with dimethyl carbonate. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0d4133dd6314ab3a6af35d9a5101b828f28a7059/2-Figure1-1.png)
Figure 1 from 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) and microwave-accelerated green chemistry in methylation of phenols, indoles, and benzimidazoles with dimethyl carbonate. | Semantic Scholar
![The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity. | Semantic Scholar The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7f11e50e3c561e40fc500f0bf3918481d2ad6938/1-Figure1-1.png)
The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity. | Semantic Scholar

DBU, 1,8-diazabicyclo 5.4.0 undec-7-ene, is a base in elimination reactions. Which N atom is more basic in DBU? Explain your choice. | Homework.Study.com
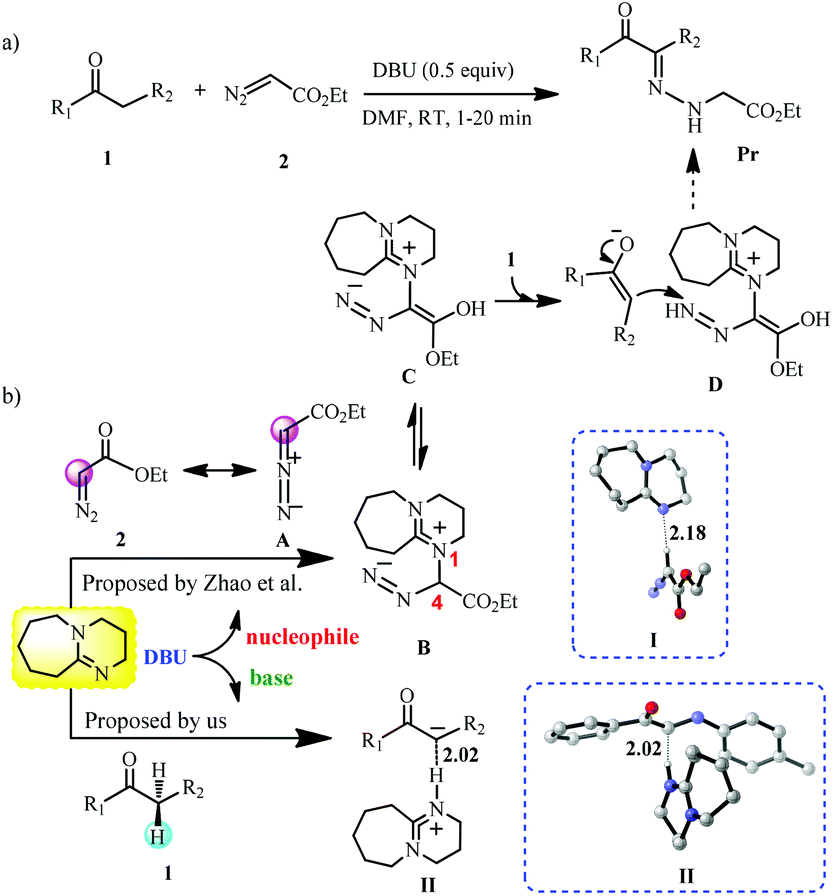

![DBU [1,8-Diazabicyclo[5,4,0]undec-7-ene] - An Overview - YouTube DBU [1,8-Diazabicyclo[5,4,0]undec-7-ene] - An Overview - YouTube](https://i.ytimg.com/vi/fu67cE_ydew/maxresdefault.jpg)




