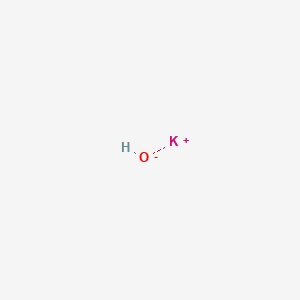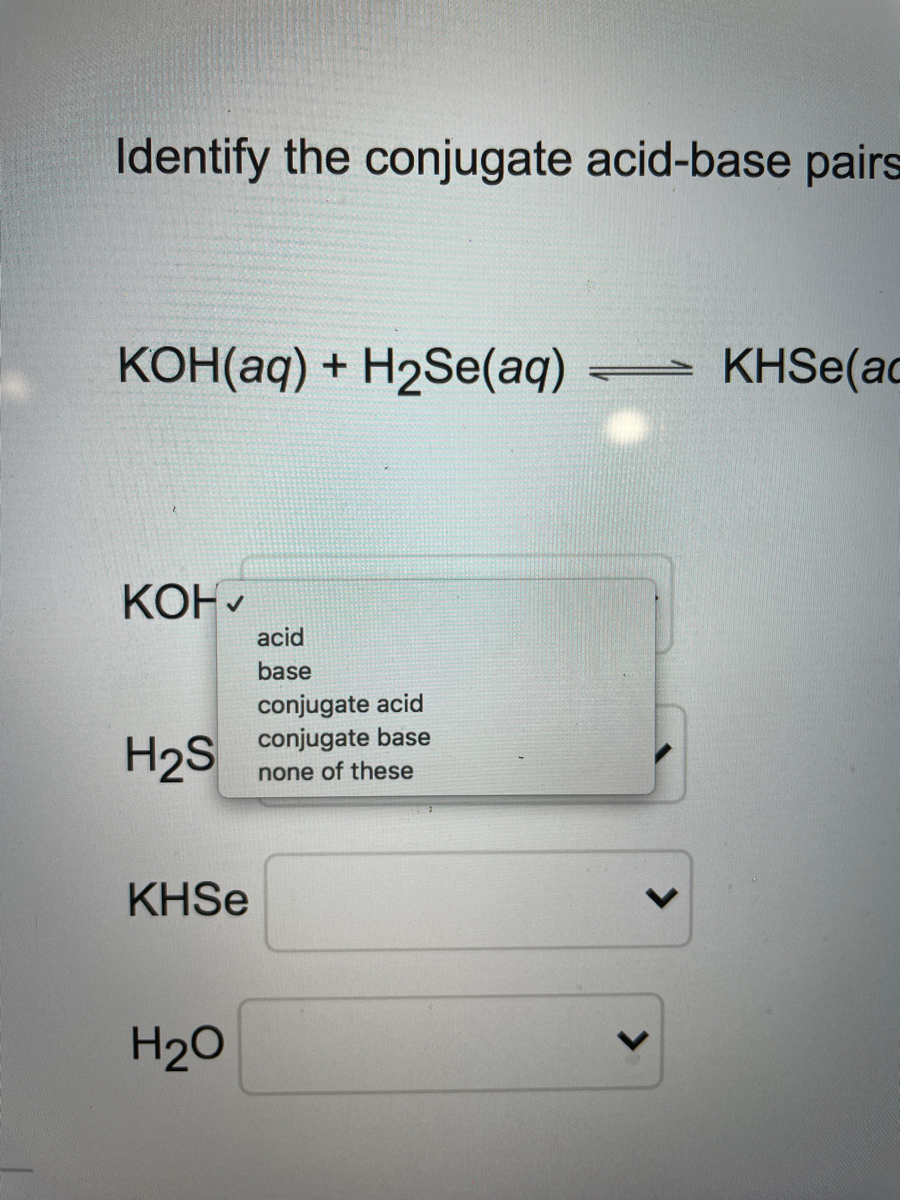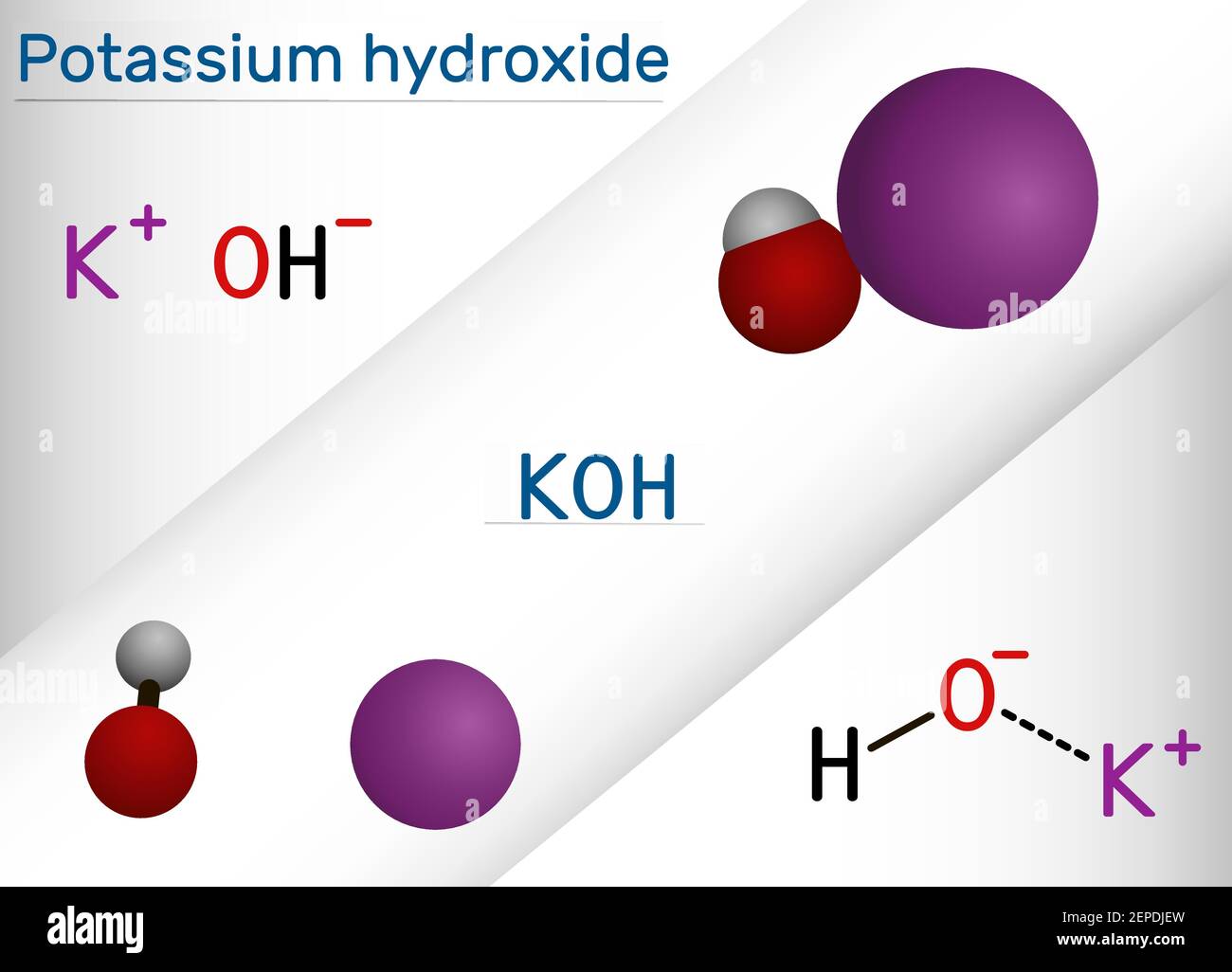
Potassium hydroxide, caustic potash, lye molecule. KOH is strong caustic base and alkali, ionic compound. Structural chemical formula and molecule mod Stock Vector Image & Art - Alamy
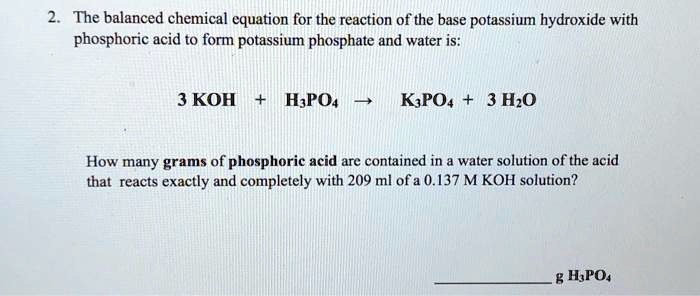
SOLVED: The balanced chemical equation for the reaction of the base potassium hydroxide with phosphoric acid to form potassium phosphate and water is: 3 KOH + H3PO4 -> K3PO4 + 3 H2O
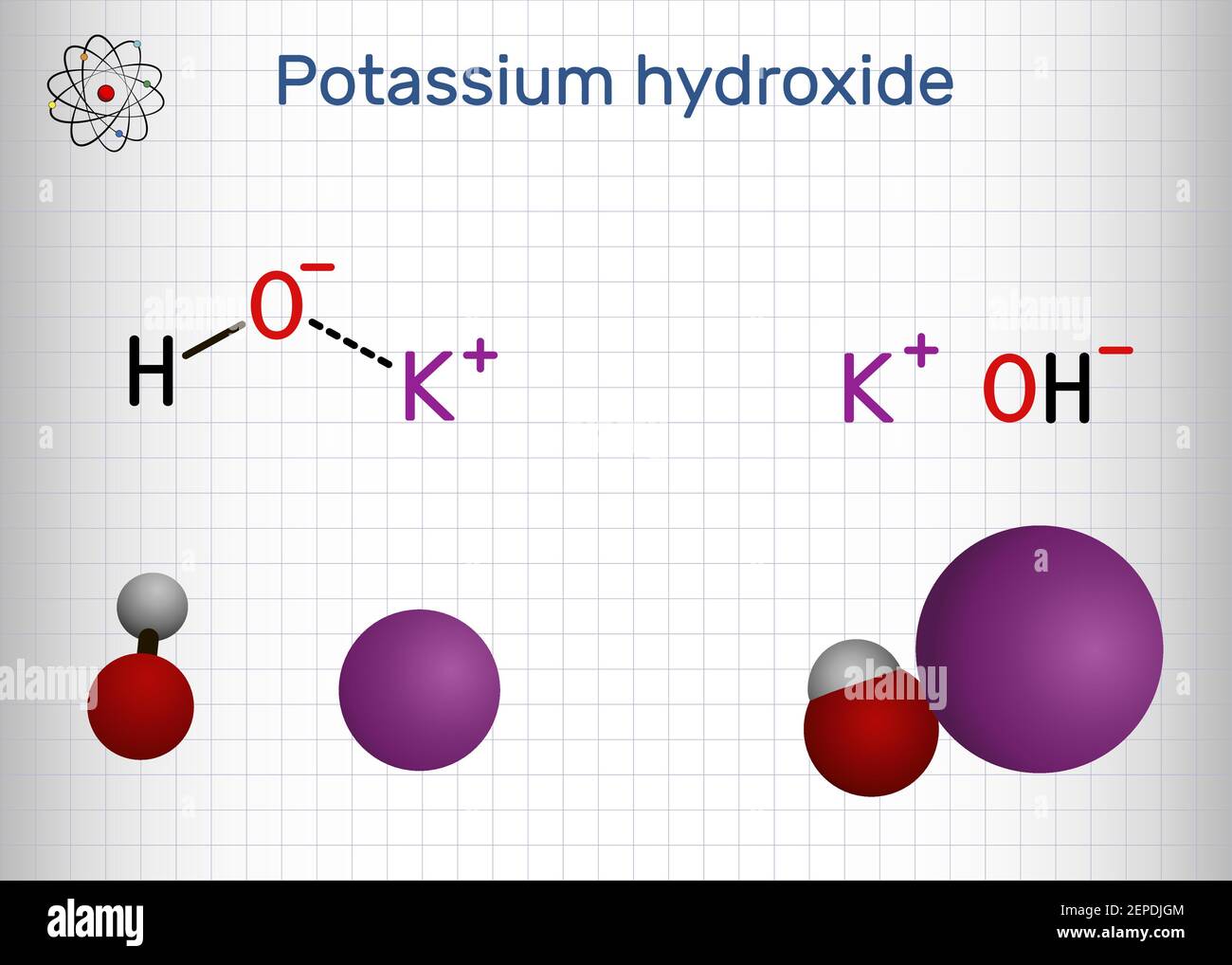
Potassium hydroxide, caustic potash, lye molecule. KOH is strong caustic base and alkali, ionic compound. Structural chemical formula and molecule mod Stock Vector Image & Art - Alamy

Write the neutralization reaction for the following acid and base: HCl_{(aq)} and KOH_{(aq)}. | Homework.Study.com
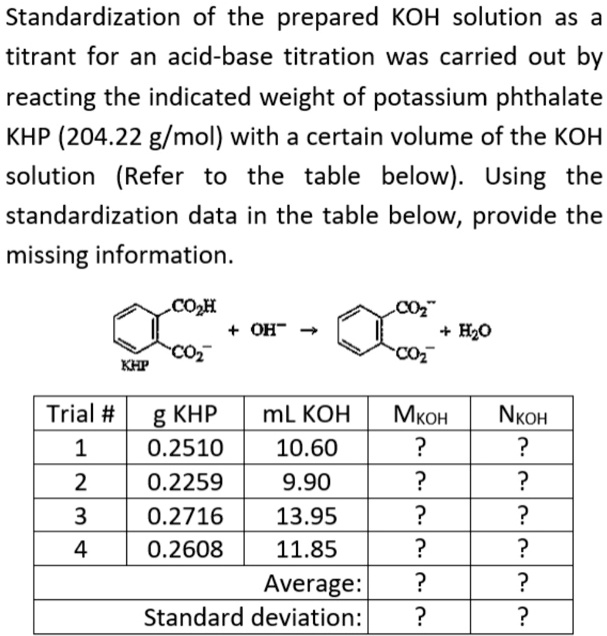
SOLVED: Standardization of the prepared KOH solution as a titrant for an acid-base titration was carried out by reacting the indicated weight of potassium phthalate KHP (204.22 g/mol) with a certain volume

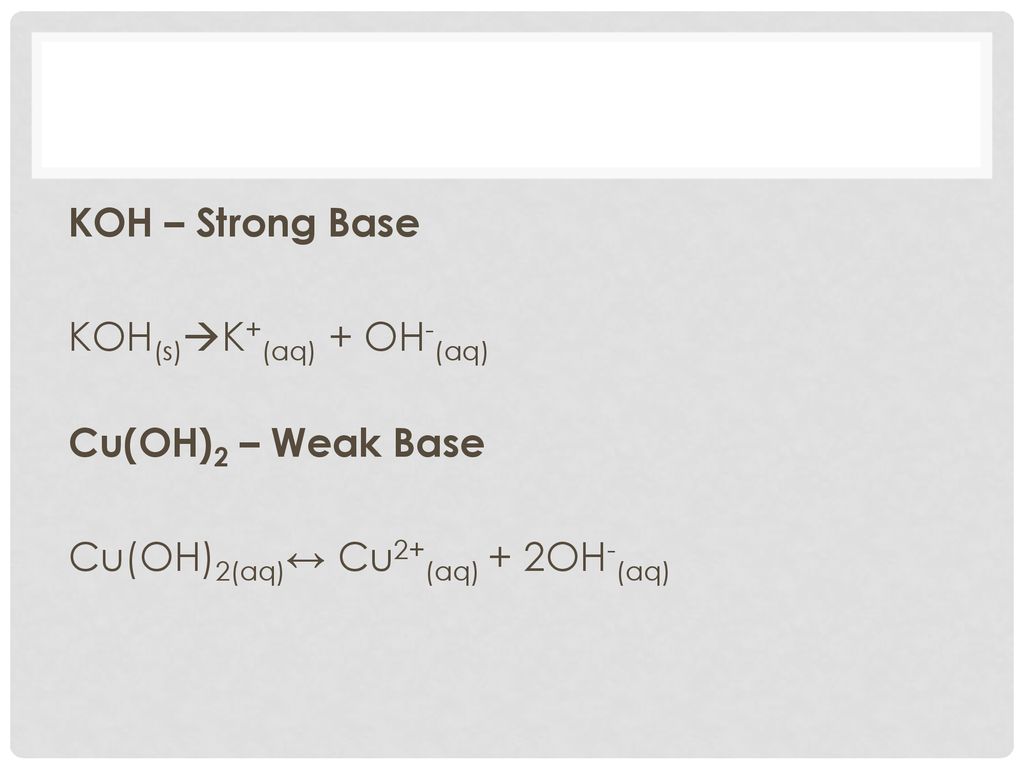
SOLVED: Potassium hydroxide dissociates in water to produce hydroxide ions. KOH(s) + H2O(l) â†' K+(aq) + OH-(aq) Given the information above, how is potassium hydroxide categorized? A. Both a Bronsted-Lowry base and
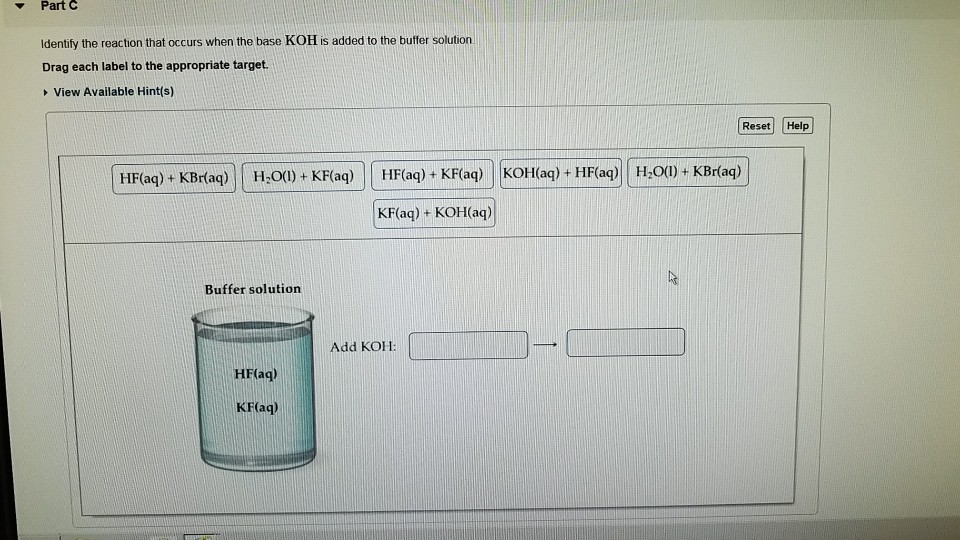
![Identify the reaction that occurs when the base KOH is added to the buffer solution. [{Image src='buffer_solution613505384566154416.jpg' alt='buffer solution' caption=''}] | Homework.Study.com Identify the reaction that occurs when the base KOH is added to the buffer solution. [{Image src='buffer_solution613505384566154416.jpg' alt='buffer solution' caption=''}] | Homework.Study.com](https://homework.study.com/cimages/multimages/16/buffer_solution613505384566154416.jpg)
Identify the reaction that occurs when the base KOH is added to the buffer solution. [{Image src='buffer_solution613505384566154416.jpg' alt='buffer solution' caption=''}] | Homework.Study.com