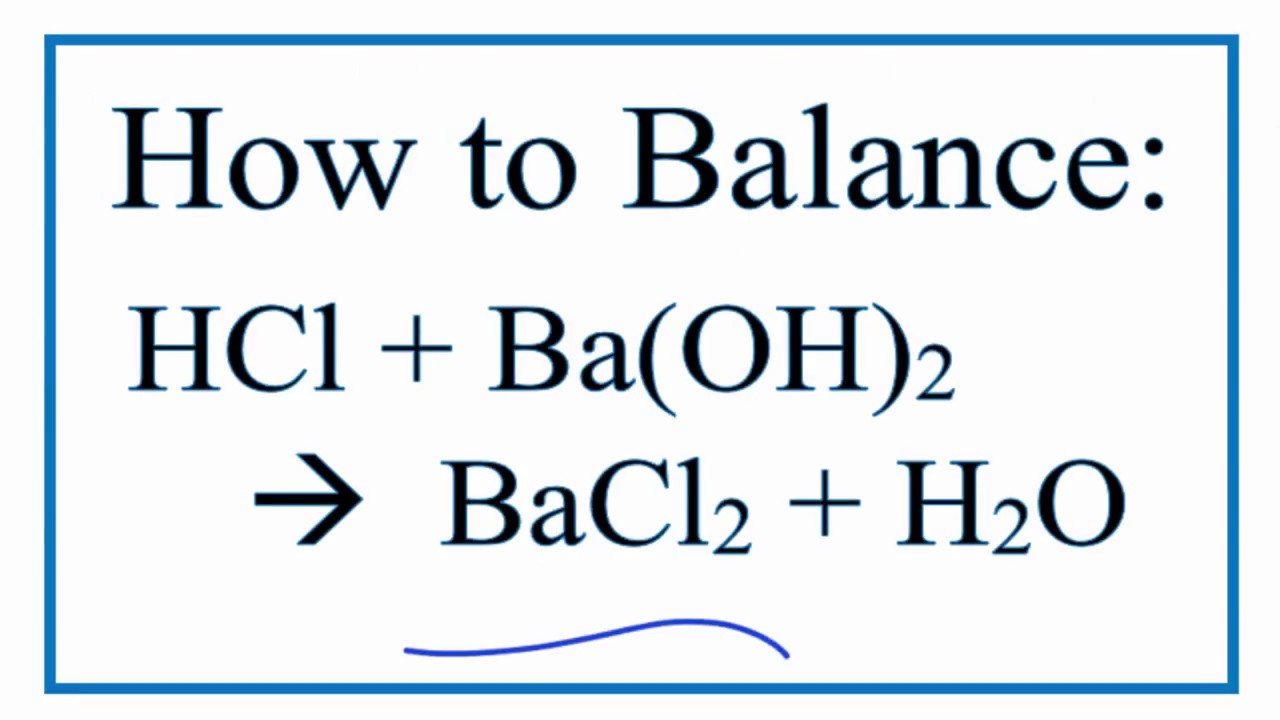
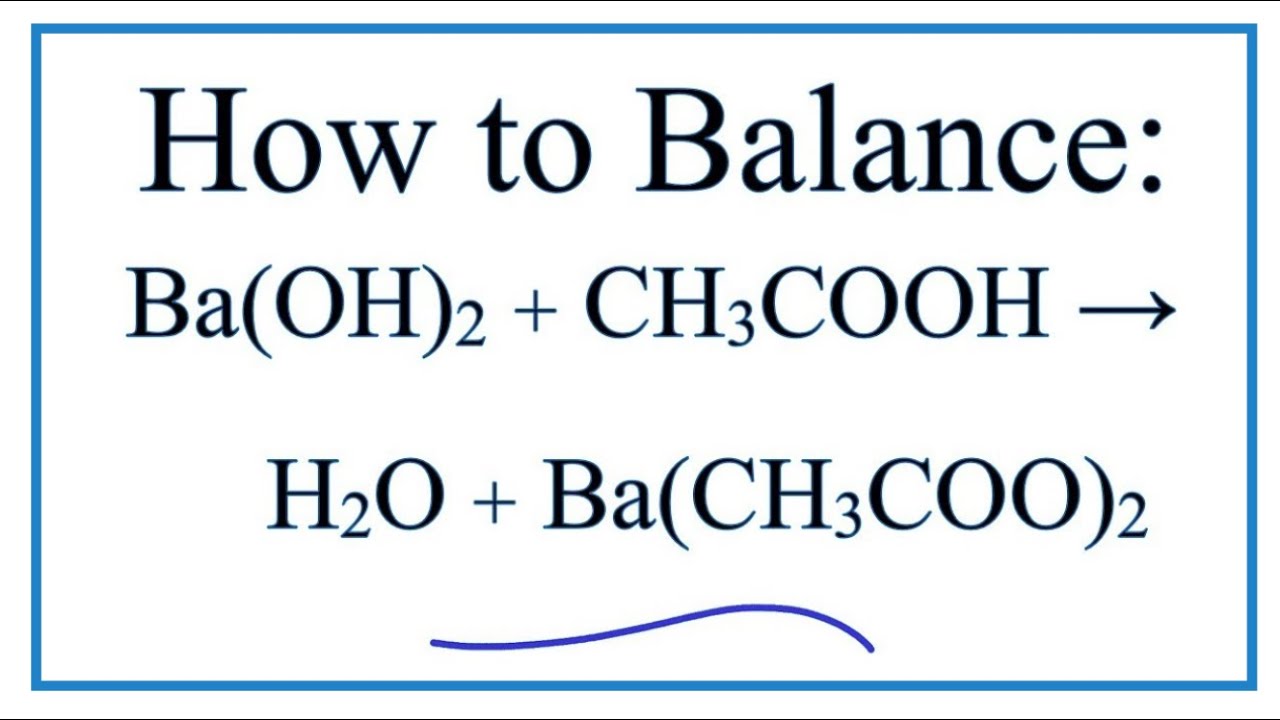
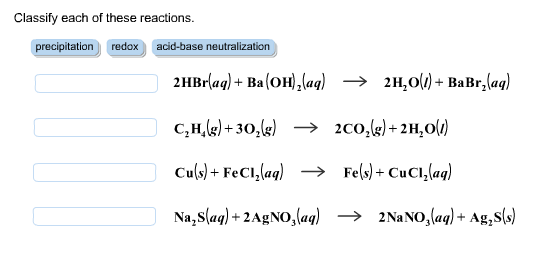
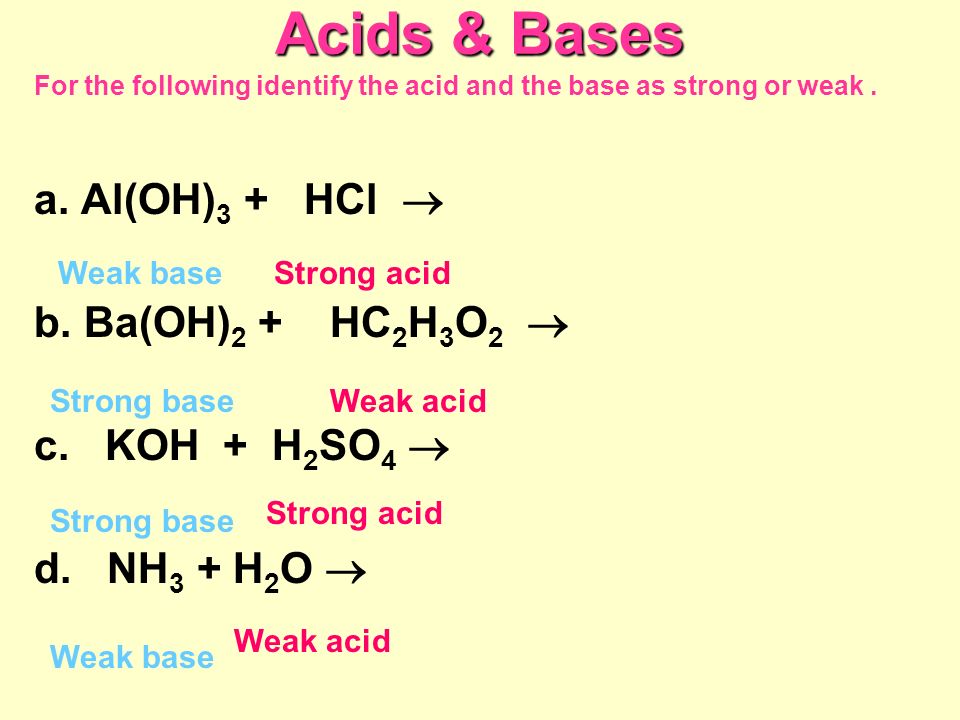
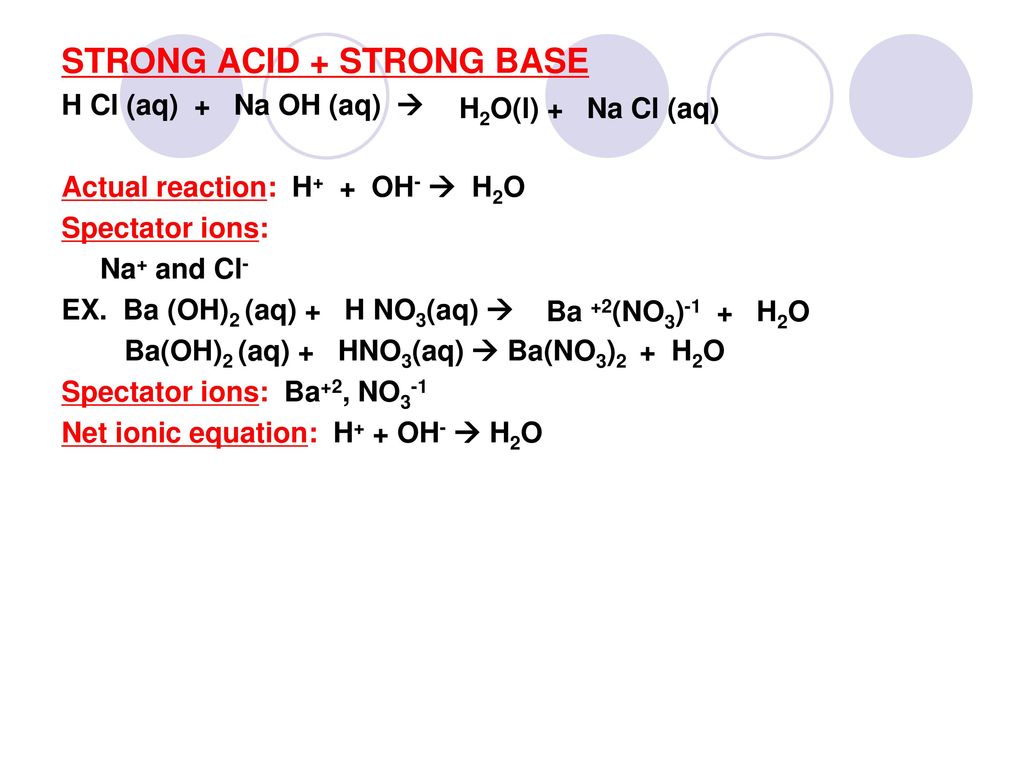
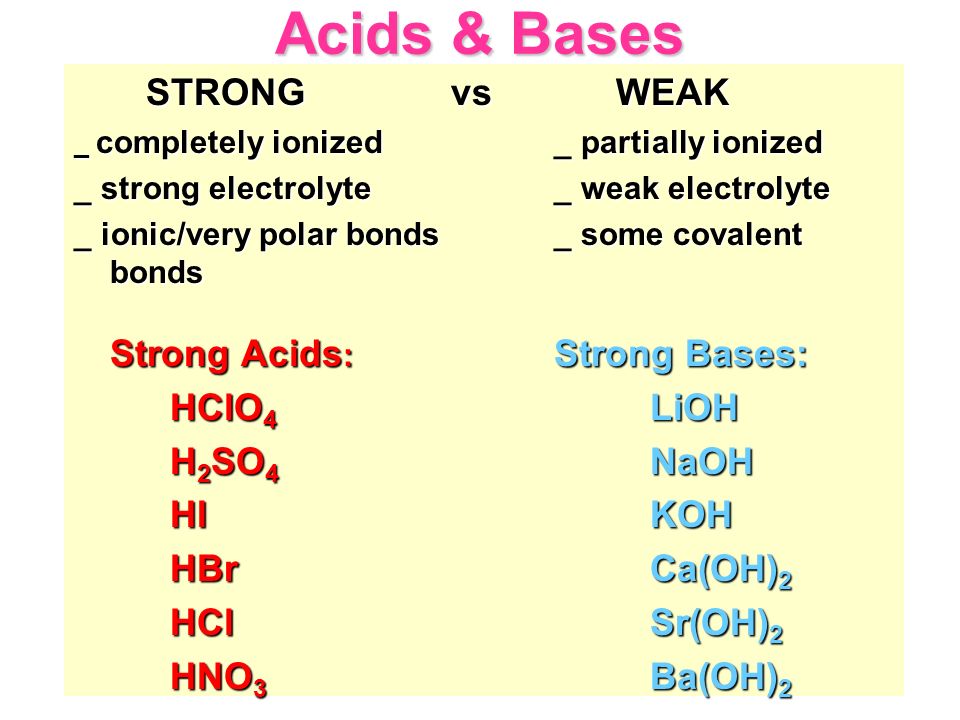
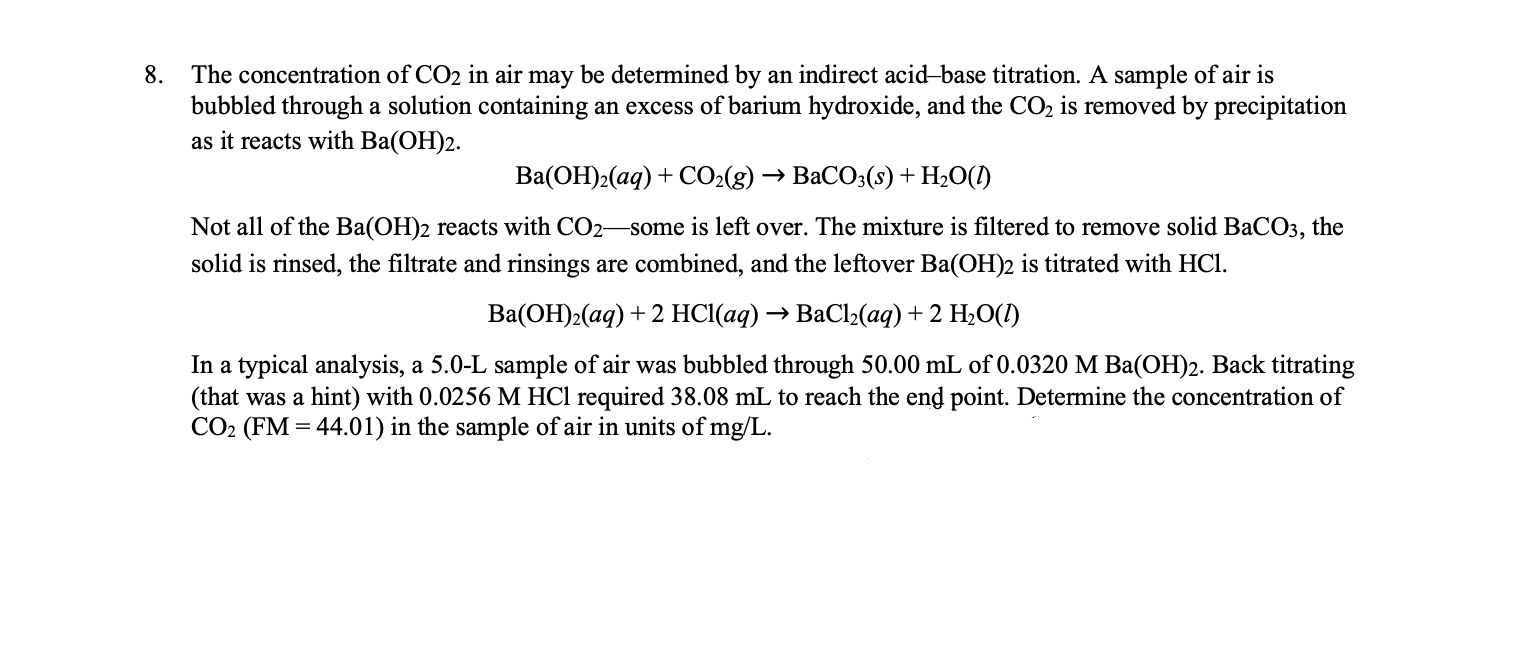
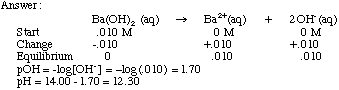Acids & Bases Acids: acids are sour tasting Arrhenius acid Arrhenius acid: Any substance that, when dissolved in water, increases the concentration. - ppt download
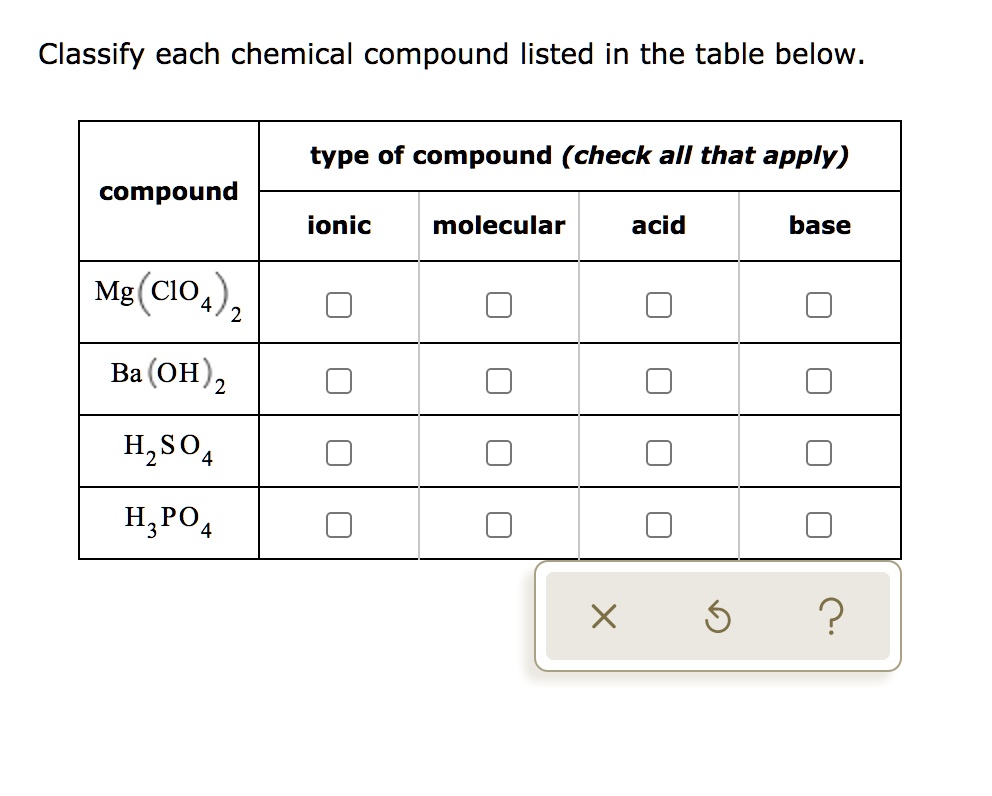
SOLVED: Classify each chemical compound listed in the table below type of compound (check all that apply) compound ionic molecular acid base Mg ClO Ba (OH) 2 H2S04 HzPO

SOLVED: Identify whether each substance is an acid or base. RbOH Choose. HCIO4 Choose . Ba(OH)2 Choose. HNO2 Choose . KOH Choose .